



|
Jens Peder Dahl was born 1934 in Denmark. He went to The Technical University of Denmark where he got his Masters in chemistry, with
biotechnology as his principal subject. Dahl obtained his Dr. under Ballhausen, spent 1.5 years with Slater at MIT, became Professor of Chemical Physics at the The Technical University of Denmark in Copenhagen in 1972, a post which he held until his retirement. Prof. Dahl has worked in Theoretical Inorganic and in Theoretical Chemistry, he is the author of many papers, books, and articles in books.
Photo at right from Dahl's Faculty Page. |

Photo courtesy and © J.P. Dahl |
| Video clip. | Dahl talks about his interaction with Ballhausen. (5 min). | MPEG4; 8 MB | There is still the problem concerning codecs and Microsoft. Especially with IE6 and Win Media Player: Under certain browser settings of IE6 that may got as far as: "File not found on the server." - it's there of course. Sad but true - the codec fight carried out on the back of the user! If you know what you are doing just go into regedit and kill the connections. | in English |
| Video clip download. | Dahl talks about his interaction with Ballhausen. (5 min). | ZIP of MPEG4 of the above; 5.5 MB | While IE6 now apparently ckecks all files as to their contents before downloading (see above) them, it so far (May 2002) doesn't dare to do this to ZIP files. Which means that you can download it, unzip it, and then view it. I found out that this presently is the best way handling MPEG without the threatening behaviour of Microsoft and IE6. | in English |
| Sound clip | Dahl talks about his interaction with Ballhausen. (5 min). | WAV; 3.5 MB | The sound-only track of the above videos. | in English |
| Sound clip | Dahl talks about his interaction with Ballhausen. (5 min). | MP3; 730 KB | Maybe here the same trouble with IE6 and the Win Media Player. | in English |
| Text of the above. | Dahl talks about his interaction with Ballhausen. | HTML | in English |
| J.P. Dahl : Quantum Chemistry, Fundamentals, 1996.
Forword (in Danish). |
J.P. Dahl :
Introduction to the Quantum World of Atoms and Molecules, 2001 (in English).
"This invaluable book provides a balanced and integrated introduction to the quantum world of atoms and molecules. The underlying basis of quantum mechanics is carefully developed, with respect to the historical tradition from a molecular angle. The fundamental concepts in the theory of atomic and molecular structure are thoroughly discussed, as are the central techniques needed in quantum chemical applications. Special attention is paid to exposing and clarifing the common ground of Hartree-Fock theory and density-functional theory. Throughout the text, the discussion is pedagogically obliging and aims at simplicity and mathematical clarity, while avoiding the use of advanced mathematics. End-of chapter problems supplement the main text." This website agrees with this description -> a book from a chemist for chemists (after all, most chemists are concerned with substances, although some (fortunately) tend to be inclined to more theoretical concepts! Another way to use this book would be to make it a basis for a surveying pre-course of the type "What you have to expect and watch out for in your quantum chemistry." Because it's also a polite and friendly book, with a low frustrating level, which aims at the young student. |
|
A good inorganic theoretical chemistry classic, hard to get, is : J.P. Dahl and C.J. Ballhausen, The Electronic Structure of Ferrocene Mat. Fys. Medd. Dan. Vid. Selsk. 33, no. 5, 1-23 (1961) ......... here (1 chunk= 1.6MB) Or And then it is cut into 21 pages, 80 KB average, at this place ...... here. |
| D: Very little, I must say. This was very elementary mathematics. We did have to learn a little about matrices and matrix theory, but not very much, and very little about differential equations and so on. So it was a quite poor mathematical education, by today's standards certainly. But I was myself mathematically inclined, and quite early did I become interested in quantum mechanics. So while I was still studying for my master's, I would often sit down at night after having prepared myself for the next day, and go through the mathematics books which the students of mechanical and electrical engineering used. They were very good textbooks, written by
Harald Bohr,
a brother of
Niels Bohr, together with A. F. Andersen and Richard Petersen. I systematically worked through those volumes, and so I learned the mathematics essentially by myself. A: And then, as you said on your video conversation, you switched to Ballhausen. That meant that you switched from the Technical Highschool to the University? D: Well, not quite. For as I said, the Department of Physical Chemistry served both the University and the Technical Highschool. So, actually my Ph.D. degree was a degree at the Technical Highschool, but the work was carried out at this University department. This was a good tradition which we had in Denmark at the time, that several professors of chemistry taught both at the University and at the Technical Highschool. |
 in 1972 Photo courtesy and © J.P. Dahl |

Photo taken from here. |
D: Well, first a little about the Department of Physical Chemistry at that time. It is obvious that Ballhausen played the central role in the Department. But there was also another young theoretical chemist by the name of
Thor Bak who really should be mentioned, because he was a very inspiring teacher. I had, in fact, a close contact with him at an early stage while Ballhausen was still in the United States. Thor Bak was a very good person for young people. And after that Ballhausen had become a professor, in 1959, the two of them tended to run much of the Department together. Thor Bak then became a professor on his own a few years later. Anecdotes … A: Or something a student might think about difficulties or overcoming difficulties when you did that work. I mean, you sat down and started to work at that. Of course there were many hindrances. You came to a point where at a sudden you found that to go beyond, you had to study something. |
| A: Was it an electromechanical calculator? D: Yes, and it could perform additions, subtractions, multiplications and divisions. But when you needed a square root, you would have to generate it by an iterative procedure. Later I got a Friden electronic calculator, on which you could work just like you work on a typewriter. It had the property that it could extract square roots which was a great thing but, of course, cosines and so on it couldn't do - but a square root! A: This was in 1961, and then … |

Photo and © taken from here. |
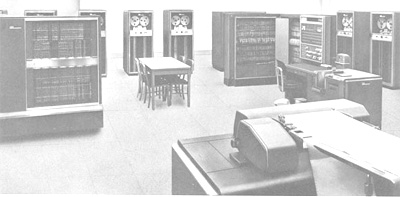 |
Photo and © taken from here. |

Photo and © taken from here.  Punched tape and roll Watch the full tape roll in the left hand. Photo and © taken from here. |
D: No, that was with cards. But as I said, after I got my Ph.D. I spent two years with the Navy. During my second year, I carried the rank of lieutenant and was asked to work at the computer DASK, doing calculations on mine sweeping and torpedo trajectories. The Danish Navy had, in part, paid for this first Danish computer called DASK which was placed on the ground floor of a villa belonging to
Carlsberg, it sort of filled this whole ground floor. It was based on tubes of course. And there you had to use paper tapes with only five holes across. It was produced on a so-called Flexowriter, and you had to type correctly to punch the holes. You also had to press all the buttons on the computer yourself. A: And once the tape broke, you had to retype? D: No, you could cover the mispunched holes with adhesive tape and then manually punch some new holes. That was possible. So, first I had an electric calculator, then I worked with this pioneer-type computer, and then at MIT, of course, with more advanced computers. |

Notice the paper tape the person just is checking Photo and © taken from here. |

Photo and © from his faculty page. |
D: Well, some years later, I must say. A: And at the Technical Highschool, could you do any calculation you wanted, or did you also have students who worked on Technical Highschool matters? Were you free to … D: Well, when I came to the Technical Highschool in 1972 as a professor this was a new chair, although I had already served as an external lecturer for about three years before that. I had been giving lectures in quantum chemistry. And those were quite popular lectures. The students liked them and they thought this was no more difficult than physical chemistry when you came down to it. Soon, I could accept a couple of students as Ph.D. students, and one of my coworkers at the University, Helge Johansen, joined me as a lecturer. During the years, we supervised several students together. And we always worked on our theoretical ideas and our theoretical projects. We did calculations and we studied group theory and various aspects of quantum chemistry. We were not in any way asked to concentrate on problems related to technical chemistry. |
Photo and © from his faculty page. |
D: Well, they approached of course Ballhausen, because he was the senior person. So he came to me and said: "I have this invitation, and I would be happy if we could work on it together". So I started working on it and so I actually wrote most of it. But then, when I came to the permanganate ion I said: "Well, I think you should write this". "Of course", he said, and he went on and wrote it. But as it is, as a younger person I was the one who did most of the work. But I think we did reasonably well, at the time at least. A: And the crystalline perturbation of the magnetic dipole transition in Ni(CN)42- … D: Oh yes, that was the work done together with Ray Dingle and Martin Vala. What we had here were square planar ions stacked on top of each other in a crystalline surrounding, but with successive ions rotated with respect to each other about the four-fold axis, with the angle of rotation depending upon the cations in the crystal. The centre of the absorption band associated with the weak electronic transition that we studied was highly dependent on this angle of rotation, and we were able to account for the details of this dependence by means of perturbation theory, and at the same time we could verify that the polarisation of the transition was that of a magnetic dipole transition. Yes, this was an interesting piece of work. |
| D: Let me put it this way: I never completely left the fields of MO theory, because I always had Ph.D. students who worked on transition metal compounds, on symmetry and on electron-electron correlation. For instance, Irene Shim and I became interested in studying the diatomic nickel molecule, Ni2, to see what is Ni2, how is that built up as a sort of precursor for the Ni crystal . This was work we did in the seventies, because it was not really known how you come from one atom to two atoms and so on, over mesoscopic systems to crystals, a theoretical process that has been much studied by several groups later. For Irene Shim, the work on Ni2 became the beginning of a long series of papers on diatomic molecules built of metal atoms. | Photo and © from his faculty page. |

Photo and © from his faculty page. |
I would also like to mention the extensive work on electronic structure and the permutation group and unitary groups, done in the nineteen seventies and later by
Sten Rettrup
and C. R. Sarma, and also the fine work on Coulomb and Fermi correlation by Xuejun Feng.
But it is true, I always had an inclination to become interested in quantum mechanics basically, an inclination to look at things from different angles and to find alternatives to things, to the way you look at a problem, because quantum mechanics is a rich and very complicated theory. You look and consider wave functions in position space and wave functions in momentum space, but you have to sort of combine these two pictures into a single mental picture in order to understand the correlation between momentum and position. And then, as I mentioned, I was always interested in symmetry. So, of course, I was also interested in the symmetry of the hydrogen atom, and I cultivated this interest at the same time as I did electronic structure calculations, already while I worked in Ballhausen's group. Ballhausen, of course, was aware of this and supported me when I started to study the Kepler problem and spin problem and so on. Let me remind you of the nature of the special symmetry associated with the Kepler problem: For any atom the three 2p-orbitals are degenerate, and so are the five 3d-orbitals. This degeneracy has to do with the three-dimensional rotation group. |
|
 Photo and © from X. Feng's private page. |
